Varroa Mite Sampling Methods

VARROA MITE SAMPLING METHODS
From the Extension Fact Sheet of the same title (linked to the right)
Authored by Morgan A. Roth, Aaron D. Gross, and James M. Wilson Department of Entomology, Virginia Tech
Varroa mites are one of the greatest threats faced by beekeepers today. This fact sheet will explore Varroa mite sampling methods, which are critical to effective Varroa management. To learn more about Varroa mite biology how to treat Varroa mite infestations, see the accompanying Varroa Mite Biology and Feeding Damage and Varroa Mite Management Methods fact sheets.
Over the years, four prominent sampling methods have been developed for estimating Varroa mite infestation levels. Two of these methods, drone brood removal and sticky boards, are qualitative measures and are considered less reliable due to difficulties interpreting mite population levels. The other two methods, the alcohol wash and powdered sugar shake, are quantitative methods, which yield more accurate population estimates. An accurate population estimate is crucial when making informed management decisions about varroa mite management. These methods will be presented in order of most invasive to least invasive qualitative and quantitative sampling methods.
DRONE BROOD REMOVAL: This method is highly invasive and directly samples brood by uncapping and removing drone brood with a cappings-scratcher. Once the brood are removed, they can be individually examined, and the number of adult female Varroa mites per larva or pupa are recorded.
STICKY BOARDS: This method is least invasive and uses a sticky board to catch fallen mites. The IPM sticky boards are made with a grid system to help show spatial distribution and is placed beneath the bottom hive body to catch dead or fallen mites over time. The sticky board is separated from the rest of the hive by a screened bottom board, which also helps improve hive ventilation, although the screened bottom board itself is a cultural control that is not always associated with sticky board sampling. Sticky boards can be purchased commercially, but can also be homemade using petroleum gel, or other clear adhesive substances. Sticky boards are often used in conjunction with an acaricide treatment, which causes the mites to fall more quickly. Subsampling from the grid on the sticky board (based upon spatial patterning) can be the most efficient method when large numbers of mites are collected, with population estimate methods depending on grid configuration. However, this method works better as a way to check treatment effectiveness before and after treatment than to actually obtain a mite population estimate.
ALCOHOL WASH: The other highly invasive, though popular and precise, method for estimating V. destructor infestation is the alcohol wash. Although procedures differ as to the number of worker bees to collect, 200 (±25) bees can be gathered by tapping a frame of primarily capped brood into a plastic container (Figure 1).
Figure 1. A frame of capped brood is tapped in a plastic container for easy nurse bee collection.

A 1⁄4 cup measuring cup is then used to scoop bees into a jar for collection (Figure 2). Frames of capped brood are used because Varroa mites in the travelling stage are found to be most prevalent on nurse bees since they have frequent access to brood. Rubbing alcohol can then be added to the jar, which is then shaken for at least 1 minute. The alcohol is then poured out of the jar, along with mites that were on the bees. The number of mites can be divided by two, giving the number of mites per 100 bees, based upon the 200 bees estimated to be in the 1⁄4 cup of bees. This number is reported as the percent infestation, and thresholds are based upon this metric. Thresholds range between 2-5% based upon the season and colony cycle, and treatment is recommended when infestations exceed this threshold.
Figure 2. 1⁄4 cup of bees is added to the jar (200±25 bees).

POWDERED SUGAR SHAKE: The powdered sugar shake combines the benefits of alcohol wash accuracy with the fact that it is a non-invasive sampling method. Replacing alcohol with powdered sugar eliminates bee death in the sampling process and does not impact bees used in sampling long term. During the development of this method, various inert dusts, including talcum powder, wheat flour, baking soda, corn starch, and fine sugar, were all tested; however, the greatest accuracy in mite detection was achieved with powdered sugar. Bees are, again, collected with a 1⁄4 cup measuring cup, and are deposited into a jar with a 1/8 inch mesh-topped lid. Bees are then shaken in 1-2 tablespoons of powdered sugar for 1 minute (Figure 3), allowed to rest for one minute, then the jar is inverted and shaken for another minute into a collection tray so that mites can be counted (Figure 4).
Figure 3. One to two tablespoons are added through a mesh topped lid prior to shaking.

This method is now most highly recommended to beekeepers because of its high levels of accuracy and nondestructive sampling procedures. Although this method does not guarantee that all mites were removed during shaking, this method is the best way to gather a Varroa mite population estimate without damaging the hive.
Figure 4. Varroa mites are shaken into a collection tray during the final step of the powdered sugar shake.

Regardless of the sampling method used, it is important to sample before any treatments are applied to the hive. A sampling can then follow after treatment, which gives a good indication of how effective the treatment was. Periodically checking mite infestation levels throughout each colony phase can help ensure that colonies are treated efficiently, should the need arise.
USEFUL REFERENCES:
Honey Bee Health Coalition. 2018. Tools for Varroa Management a Guide To Effective Varroa Sampling & Control
https://honeybeehealthcoalition.org/wp- content/uploads/2018/06/HBHC Guide_Varroa_Interactive_7thEdition_June2018.pdf.
Gary S. Reuter and Marla Spivak. University of Minnesota Instructional Poster #155, Department of Entomology. https://articles.extension.org/pages/22279/powder-sugar- roll-for-varroa-sampling.
This work has been made possible in part by funding from Sustainable Agriculture Research and Education On-Farm Grant # OS18-122
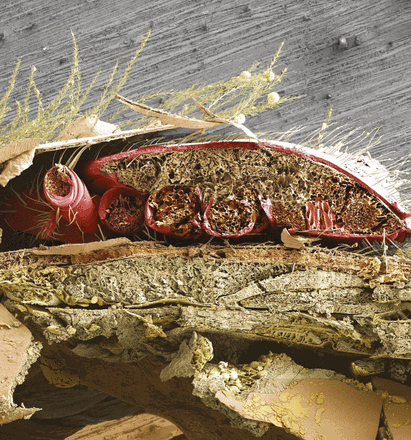
Pictured is a cross-section of an adult honey bee’s abdominal region showing a parasitic Varroa destructor mite wedged between the overlapping abdominal plates. Samuel D. Ramsey et al. found that V. destructor damages honey bees by consuming fat body, a tissue analogous to the mammalian liver, rather than by consuming hemolymph as previously believed. This finding explains the diversity of Varroa-associated pathologies and may help develop control strategies for V. destructor, which is a major driver of honey bee decline. See the article by Ramsey et al. on pages 1792–1801. Image courtesy of Samuel D. Ramsey (University of Maryland, College Park, MD), Gary Bauchan, and Chris Pooley (Agricultural Research Service, United States Department of Agriculture, Beltsville, MD).